It has been seen that the decreasing of the temperature below 200K induces another transformation to a new phase called N-phase.
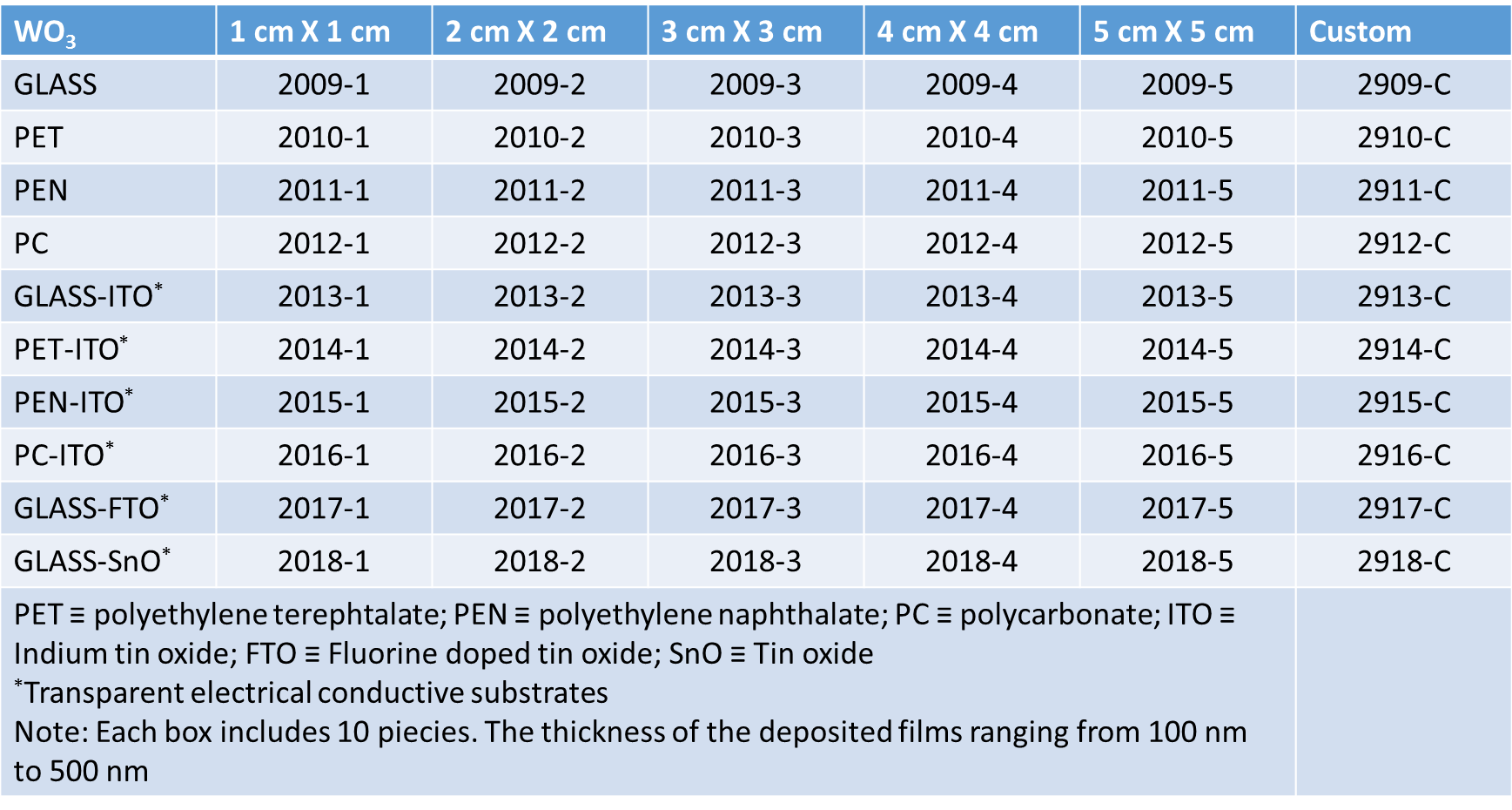
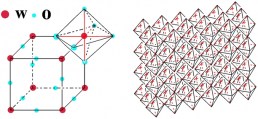
Substoichiometric phases (Magneli phases) with edge sharing octahedra are reported for tungsten oxide. These phases have the chemical formula
WO3-z with z>0 and the structure can be express as WmO3m-1 and WmO3m-2 (m N).
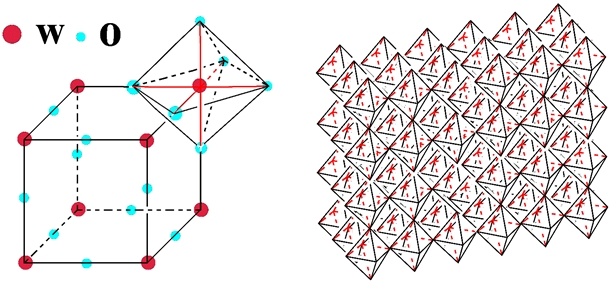
Defects as shear planes, pentagonal bipyramidal columns and hexagonal tunnel have been found: the corner-sharing WO6 octahedra contain defects with hexagonal and pentagonal cross sections. The hexagonal phases of WO3 is at the basis of its electrochromic behaviour. In fact, the “empty area” between the WO6 octahedra is so large that it can contain ions and for this reason the tungsten oxide structure is a good ions intercalation / deintercalation host.
If a metal of the first column of the periodic table (M) is intercalated in the WO3 host the results will be a specie MxWO3, known as tungsten bronzes. The crystalline structure of the tungsten bronzes depends on the type and on the density of the added species to the WO3 host. It has been seen that for lithium ions intercalation the crystalline structure pass from monoclinic to tetragonal and after to cubic as the x values increases and vice versa, also if the changing of the phase does not occur at the same values of x. However, hexagonal structures for LixWO3 have been reported.
The sites present in the WO6 octahedra network in the cubic structure are available only for small ions (H+, Li+ and Na+) while those present in the tetragonal structure are large enough (tunnels) for include K+. Finally, the hexagonal structure allows bigger ions as Rb+, Cs+ to be accommodated and the presence of trigonal tunnels (in the hexagonal structure) can contain lithium ions. Tungsten trioxide thin films with all the tungsten atoms in the oxidation state (VI) are transparent. After the electrochemical intercalation of xM+ (0
A low x values, the films have an intense blue colour, due to the photo intervalence charge transfer between adjacent W(V) and W(VI) atoms. Higher x values lead to the tungsten bronzes with red or golden colour; this process is irreversible.
The intervalence transitions are responsible of the broad and intense absorption band in the UV, visible or near IR regions with high values of molar extinction coefficients; for example, ε for HxWO3 is ranging from 1.4 to 5.6 dm3 mol-1 cm-1, dependently of the x values. The intervalence transitions occur when both the oxidation states of tungsten are present (W(VI) and W(V)) in the films; so complete reduction of W(VI) does not lead to an increased absorption band but on the contrary, the strong interaction between the metallic centres delocalises the electrons in a metallic-like conduction band and metallic optical properties, i.e. reflection, replace the optical absorption (generally x> ca. 0.3).
Tungsten trioxide is one of the most studied electrochromic materials and its applications do not regard only the visible region but also the IR region: determining factor for smart windows technologies.